Not generated.
1. How does ultraviolet radiation produce ozone?
theoretically, Photon energy higher than bond energy can break chemical bonds. So the wavelength is shorter than 243 nm Ultraviolet radiation can stimulate oxygen in air or water to produce ozone. Wavelength higher than 243 nm of UVC-LED Does not produce ozone.
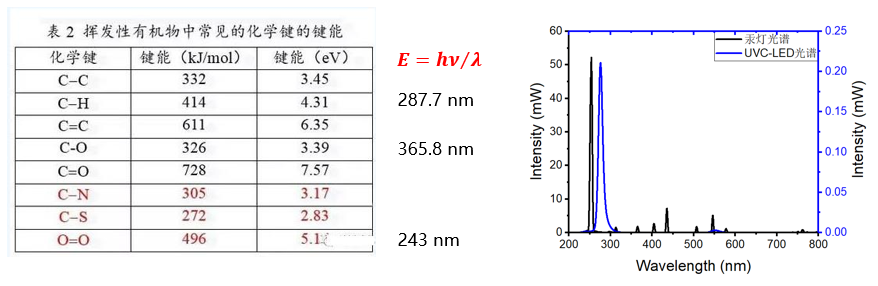
Low pressure mercury lamps produce ozone, Because the vapor pressure is 1. 3~13P Natural presence in the luminescence spectrum of low-pressure mercury lamps 185nm Vacuum ultraviolet radiation. This type of ultraviolet radiation will stimulate oxygen in the air to undergo chemical reactions and become ozone. This is essentially because ultraviolet light breaks the covalent bonds between oxygen and oxygen molecules, And then combined with oxygen molecules to form O3. UVC-LED Will not produce ozone, Because the covalent bond between oxygen and oxygen can reach up to 5. 1eV, The corresponding wavelength needs to be at least shorter than 243nm Only ultraviolet rays can interrupt it, Ultimately generate ozone.
2. LED Why doesn't it produce ozone?
Low pressure mercury lamps increase the energy level transition probability of short wave ultraviolet radiation by controlling vapor pressure, But it will also be accompanied by other transitions, with 254 and 185nm Mainly emitting light. UVC-LED The probability of first level transition is predominant, So the luminescence is pure, common UVC-LED Wavelength higher than 255 nm.
Low pressure mercury lamp is a gas light source, Its excited state energy level position is already determined by the substance mercury. So although there is high voltage, medium voltage, low pressure mercury lamp, But the difference in vapor pressure mainly only regulates the probability of transitions between different excited states, That is to say, the relative intensity of different characteristic peaks. So the low-pressure mercury lamp 254 and 185nm Mainly emitting light. UVC-LED As a quantum well structure AlGaN light emitting diode, E0 Transition dominates, There is only one peak wavelength, for example 275nm, can be found, deep ultraviolet LED The full width at half maximum of the main peak in the spectrum is significantly lower than that of blue light, It is also because quantum well luminescence has a more uniform electronic transition compared to multi size quantum dot luminescent devices. Because currently available for mass production UVC-LED The emission wavelength has not yet fallen below 243nm of, So deep ultraviolet is used for sterilization and disinfection UVC-LED, Does not produce ozone.